In the previous section, we saw the nomenclature of organic compounds carrying the aldehyde group. In this section, we will see the keto group.
• We have seen that the aldehyde group can be represented in two ways (see fig.14.63 of the previous section). In a similar way, the keto group can also be represented in two ways:
• We can use either (a) or (b) in the above fig.14.67 to represent the keto group
• There is only a small difference between the keto group and the aldehyde (shown in fig.14.67.c) group.
♦ The H atom in aldehyde group is absent in the keto group
■ Note that:
• In the hydroxyl group (ㅡOH), the bond which connects the group to a main chain starts from the oxygen atom
• In the aldehyde group (ㅡCHO), the bond which connects the group to a main chain starts from the carbon atom
• In the keto group (ㅡCOㅡ), there are two bonds which connects the group to a main chain.
♦ Both of them starts from the carbon atom
• Also there is a double bond within the group. We will see all the bonding details at the end of this section. At present, we will see the nomenclature.
• The IUPAC names of compounds with keto group end in one.
• Such compounds are called ketones.
• In any ketone, there will be a ㅡCOㅡ group
■ The naming of ketones is done by the following 2 steps:
1. Remove the 'e' from the name of the corresponding alkane
2. Put 'one' in it's place
• So we can write:
Alkane - e + one → Alkanone
• Thus we get:
♦ Methane - e + one → Methanone
♦ ethane - e + one → ethanone
♦ Propane - e + one → propanone
so on . . .
■ But there are some important points to note:
We have seen them in the case of aldehydes. We will write them again:
• The 'newly attaching keto group' has one C atom of it's own.
• So when it is attached, the total number of carbon atoms will increase by one.
• This 'total number' should be considered while naming the compound.
• This is a general rule for any functional group which carries a C atom of it's own.
♦ We applied it in the previous section for aldehydes
We can write:
■ If the functional group contains a carbon atom, that carbon atom should be treated as part of the main chain.
■ Then we will be wondering about methanone.
• Because, One hydrogen atom is removed from Methane and the ㅡCOㅡ group takes it's place to give the ketone.
• But when the group takes it's place, the total number of C atoms will become 2.
♦ So it should be named as Ethanone.
• That means, the compound methanone cannot exist. Let us analyse:
• We saw that, the aldehyde methanal does exist. We saw it's structure in fig.14.64(a) in the previous section. It is shown again in fig.14.68(a) below:
Now compare fig.14.68(a) with (b)
• In (a), the aldehyde group is shown within a magenta polygon
(i) The group has one unsatisfied bond.
(ii) We attach a hydrogen to it.
(iii) The bond is complete and we get methanal
• In (b), the keto group is shown within a magenta polygon (rectangle)
(i) The group has two unsatisfied bonds.
(ii) We attach a hydrogen to each.
(iii) The bonds are complete and we get methanal again
• So, if we try to write the structure of the simplest ketone as methanone, we will get only methanal
Let us try to make the next higher ketone. That is., ethanone:
• We saw that the aldehyde ethanal exists. We saw it's structure in fig.14.64(b) in the previous section. It is shown again in fig.14.69(a) below:
Now compare fig.14.69(a) with (b)
• In (a), the aldehyde group is shown within a magenta polygon
(i) The group has one unsatisfied bond.
(ii) We attach a hydrogen to it.
(iii) The bond is complete and we get ethanal
• In (b), the keto group is shown with in a magenta polygon (rectangle)
(i) The group has two unsatisfied bonds.
(ii) We attach a hydrogen to one.
• To the other bond, we attach a methyl radical (CH3). Because we want a total of two C atoms.
♦ But a C atom cannot be attached alone. It's other 3 valencies should be satisfied by 3 hydrogen atoms. Thus we need the CH3 group
(iii) The bonds are complete and we get ethanal again
• So, if we try to write the structure of the next higher ketone as ethanone, we will get only ethanal
■ Thus we can write:
• The simplest ketone is not methanone or ethanone, it is propanone. It's structure is shown in the fig.14.69(c) above
♦ The H atom in fig(b) is replaced by a methyl radical (CH3) in fig(c)
■ How is the propanone in fig(c) formed?
• It was originally CH3ㅡCH3. One of it's H atoms was removed and the ㅡCOㅡ group took it's place.
♦ Now there are a total of three C atoms, and so it is propanone
■ Propanone is commonly known as acetone in the industrial sector
In fact, the name 'ketone' comes from the German word 'aketone' for acetone.
■ The ketones can be represented in another way also:
• Propanone: CH3ㅡCOㅡCH3
• Butanone: CH3ㅡCH2ㅡCOㅡCH3
• Pentanone: CH3ㅡCH2ㅡCH2ㅡCOㅡCH3
so on . . .
• Note that:
♦ In the above molecular structures, the ㅡCOㅡ group is attached in the interior
♦ In the previous aldehyde group, the ㅡCHO group was attached at the end (or at the beginning)
Consider the fig.14.54 that we saw in a previous section (alcohols). It is shown again below:
• In both compounds, there are 3 carbon atoms, 8 hydrogen atoms and 1 oxygen atom.
♦In fig(a), the hydroxyl group is at the end position.
♦ But in fig(b), it is at an interior position.
• Accordingly we gave two distinct names: Propan-1-ol and propan-2-ol
■ In our present discussion on ketones also, we encounter a similar situation.
• This is because, the ㅡCOㅡ group can be anywhere in the interior of the structure
• Also note that, the group has a C atom of it's own. So the position of that C will be considered for the name.
♦ Obviously, that C should get the lowest possible number
• Let us see a solved example:
Solved example 14.8
Write the IUPAC name of the compounds given below:
(i) CH3ㅡCOㅡCH2ㅡCH3
(ii) CH3ㅡCOㅡCH2ㅡCH2ㅡCH3
(iii) CH3ㅡCH2ㅡCOㅡCH2ㅡCH3
Solution:
We will write the steps:
Case (i):
1. In the given compound, there are 4 carbon atoms. So the corresponding alkane is Butane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Butane - e + one = Butanone
3. The lowest position number is 2 from left. So we get: Butan-2-one
Case (ii):
1. In the given compound, there are 5 carbon atoms. So the corresponding alkane is Pentane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Pentane - e + one = Pentanone
3. The lowest position number is 2 from left. So we get: Pentan-2-one
Case (iii):
1. In the given compound, there are 5 carbon atoms. So the corresponding alkane is Pentane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Pentane - e + one = Pentanone
3. The lowest position number is 3 from left as well as right. So we get: Pentan-3-one
Solved example 14.9
Propanone will not require any numbering to indicate the position of it's functional group. Give reason.
Solution:
1. The structure of propanone is: CH3ㅡCOㅡCH3.
2. The ㅡCOㅡ group should be flanked by two methyl radicals.
3. If the group is at an end, there will be an 'outstanding' bond as shown below:
CH3ㅡCH2ㅡCOㅡ
OR
ㅡCOㅡCH2ㅡCH3.
4. This cannot be allowed. So the only possibility is the structure shown in (1) above
5. Since there is only one possible position for the functional group, propanone does not require any number.
Now we will see the reverse process. That is., we are given the IUPAC name of a ketone. We must write the structural formula. We will learn the method by analysing an example:
1. Given IUPAC name is: Hexan-3-one
2. Consider the word root. It is hex. So there are 6 carbon atoms
3. Consider the suffix. It is an. So the ketone is derived from an alkane
• So all the carbon-carbon bonds are single bonds
♦ alkane: ane minus e gives an
♦ alkene: ene minus e gives en
♦ alkyne: yne minus e gives yn
4. So we have 6 carbon atoms with single bonds between them.
• Note that this 'total 6 numbers' is including the C atom in the ㅡCOㅡ group.
5. So we can write:
C ㅡ C ㅡ C ㅡ C ㅡ C ㅡ C
6. The functional group is at the position 3. That is., 'carbon in the functional group' is at the position 3. So we can write:
C ㅡ C ㅡ CO ㅡ C ㅡ C ㅡ C
7. Fill all the valencies of carbon atoms.
• We use hydrogen to fill up the valencies.
• The result is: CH3ㅡCH2ㅡCOㅡCH2ㅡCH2ㅡCH3
■ In the first section in this chapter, we saw how the 'alkyl radical' gets itself attached to an open chain. See details here.
■ In another previous section we saw how a 'hydroxyl group' that is., 'ㅡOH', gets itself attached to an open chain. See details here.
■ In the just previous section we saw how an 'aldehyde group' that is., 'ㅡCHO', gets itself attached to an open chain. See details here.
■ Now we will see the same for the keto group:
• We have seen the structure of ㅡCHO group in the previous section. It is shown again in fig.14.70 (a) below:
• From fig(a), it is clear that the ㅡCHO group is in need for an electron.
♦ This need is indicated by the absence of a dot at the end of the bond line attached to the C atom.
• Now consider fig.14.70(b). It shows the bonding details in the ㅡCOㅡ group
♦ All the valency needs of oxygen atoms is satisfied (6 cyan dots plus two green dots, giving a total of 8 dots.
♦ Those of carbon is not fully satisfied. (4 green plus 2 cyan dots give a total of only 6)
• The need for two electrons is indicated by the absence of a dot at the end of each of the two bond lines attached to the C atom.
♦ Thus the group as a whole, is in need for two electrons.
• This need will be satisfied when the group gets itself attached to a hydrocarbon chain as shown in fig.14.71 below:
• Thus we get a stable molecule
• In fig.14.71, the group ㅡCOㅡ is flanked on the left by ethyl group and on the right by methyl group. So we can write: CH3ㅡCH2ㅡCOㅡCH3.
• It is Butan-2-one
In the next section, we will see the Carboxylic acid group.
• We have seen that the aldehyde group can be represented in two ways (see fig.14.63 of the previous section). In a similar way, the keto group can also be represented in two ways:
 |
| Fig.14.67 |
• There is only a small difference between the keto group and the aldehyde (shown in fig.14.67.c) group.
♦ The H atom in aldehyde group is absent in the keto group
■ Note that:
• In the hydroxyl group (ㅡOH), the bond which connects the group to a main chain starts from the oxygen atom
• In the aldehyde group (ㅡCHO), the bond which connects the group to a main chain starts from the carbon atom
• In the keto group (ㅡCOㅡ), there are two bonds which connects the group to a main chain.
♦ Both of them starts from the carbon atom
• Also there is a double bond within the group. We will see all the bonding details at the end of this section. At present, we will see the nomenclature.
• The IUPAC names of compounds with keto group end in one.
• Such compounds are called ketones.
• In any ketone, there will be a ㅡCOㅡ group
■ The naming of ketones is done by the following 2 steps:
1. Remove the 'e' from the name of the corresponding alkane
2. Put 'one' in it's place
• So we can write:
Alkane - e + one → Alkanone
• Thus we get:
♦ Methane - e + one → Methanone
♦ ethane - e + one → ethanone
♦ Propane - e + one → propanone
so on . . .
■ But there are some important points to note:
We have seen them in the case of aldehydes. We will write them again:
• The 'newly attaching keto group' has one C atom of it's own.
• So when it is attached, the total number of carbon atoms will increase by one.
• This 'total number' should be considered while naming the compound.
• This is a general rule for any functional group which carries a C atom of it's own.
♦ We applied it in the previous section for aldehydes
We can write:
■ If the functional group contains a carbon atom, that carbon atom should be treated as part of the main chain.
■ Then we will be wondering about methanone.
• Because, One hydrogen atom is removed from Methane and the ㅡCOㅡ group takes it's place to give the ketone.
• But when the group takes it's place, the total number of C atoms will become 2.
♦ So it should be named as Ethanone.
• That means, the compound methanone cannot exist. Let us analyse:
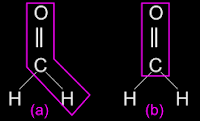
• We saw that, the aldehyde methanal does exist. We saw it's structure in fig.14.64(a) in the previous section. It is shown again in fig.14.68(a) below:
 |
| Fig.14.68 |
• In (a), the aldehyde group is shown within a magenta polygon
(i) The group has one unsatisfied bond.
(ii) We attach a hydrogen to it.
(iii) The bond is complete and we get methanal
• In (b), the keto group is shown within a magenta polygon (rectangle)
(i) The group has two unsatisfied bonds.
(ii) We attach a hydrogen to each.
(iii) The bonds are complete and we get methanal again
• So, if we try to write the structure of the simplest ketone as methanone, we will get only methanal
Let us try to make the next higher ketone. That is., ethanone:
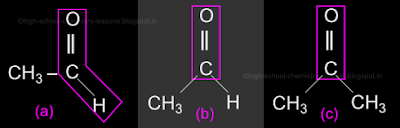
• We saw that the aldehyde ethanal exists. We saw it's structure in fig.14.64(b) in the previous section. It is shown again in fig.14.69(a) below:
 |
| Fig.14.69 |
• In (a), the aldehyde group is shown within a magenta polygon
(i) The group has one unsatisfied bond.
(ii) We attach a hydrogen to it.
(iii) The bond is complete and we get ethanal
• In (b), the keto group is shown with in a magenta polygon (rectangle)
(i) The group has two unsatisfied bonds.
(ii) We attach a hydrogen to one.
• To the other bond, we attach a methyl radical (CH3). Because we want a total of two C atoms.
♦ But a C atom cannot be attached alone. It's other 3 valencies should be satisfied by 3 hydrogen atoms. Thus we need the CH3 group
(iii) The bonds are complete and we get ethanal again
• So, if we try to write the structure of the next higher ketone as ethanone, we will get only ethanal
■ Thus we can write:
• The simplest ketone is not methanone or ethanone, it is propanone. It's structure is shown in the fig.14.69(c) above
♦ The H atom in fig(b) is replaced by a methyl radical (CH3) in fig(c)
■ How is the propanone in fig(c) formed?
• It was originally CH3ㅡCH3. One of it's H atoms was removed and the ㅡCOㅡ group took it's place.
♦ Now there are a total of three C atoms, and so it is propanone
■ Propanone is commonly known as acetone in the industrial sector
In fact, the name 'ketone' comes from the German word 'aketone' for acetone.
■ The ketones can be represented in another way also:
• Propanone: CH3ㅡCOㅡCH3
• Butanone: CH3ㅡCH2ㅡCOㅡCH3
• Pentanone: CH3ㅡCH2ㅡCH2ㅡCOㅡCH3
so on . . .
• Note that:
♦ In the above molecular structures, the ㅡCOㅡ group is attached in the interior
♦ In the previous aldehyde group, the ㅡCHO group was attached at the end (or at the beginning)
Consider the fig.14.54 that we saw in a previous section (alcohols). It is shown again below:
 |
| Fig.14.54 |
♦In fig(a), the hydroxyl group is at the end position.
♦ But in fig(b), it is at an interior position.
• Accordingly we gave two distinct names: Propan-1-ol and propan-2-ol
■ In our present discussion on ketones also, we encounter a similar situation.
• This is because, the ㅡCOㅡ group can be anywhere in the interior of the structure
• Also note that, the group has a C atom of it's own. So the position of that C will be considered for the name.
♦ Obviously, that C should get the lowest possible number
• Let us see a solved example:
Solved example 14.8
Write the IUPAC name of the compounds given below:
(i) CH3ㅡCOㅡCH2ㅡCH3
(ii) CH3ㅡCOㅡCH2ㅡCH2ㅡCH3
(iii) CH3ㅡCH2ㅡCOㅡCH2ㅡCH3
Solution:
We will write the steps:
Case (i):
1. In the given compound, there are 4 carbon atoms. So the corresponding alkane is Butane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Butane - e + one = Butanone
3. The lowest position number is 2 from left. So we get: Butan-2-one
Case (ii):
1. In the given compound, there are 5 carbon atoms. So the corresponding alkane is Pentane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Pentane - e + one = Pentanone
3. The lowest position number is 2 from left. So we get: Pentan-2-one
Case (iii):
1. In the given compound, there are 5 carbon atoms. So the corresponding alkane is Pentane
• Remove the 'e' at the end.
2. Put 'one' in it's place. We get:
Pentane - e + one = Pentanone
3. The lowest position number is 3 from left as well as right. So we get: Pentan-3-one
Solved example 14.9
Propanone will not require any numbering to indicate the position of it's functional group. Give reason.
Solution:
1. The structure of propanone is: CH3ㅡCOㅡCH3.
2. The ㅡCOㅡ group should be flanked by two methyl radicals.
3. If the group is at an end, there will be an 'outstanding' bond as shown below:
CH3ㅡCH2ㅡCOㅡ
OR
ㅡCOㅡCH2ㅡCH3.
4. This cannot be allowed. So the only possibility is the structure shown in (1) above
5. Since there is only one possible position for the functional group, propanone does not require any number.
Now we will see the reverse process. That is., we are given the IUPAC name of a ketone. We must write the structural formula. We will learn the method by analysing an example:
1. Given IUPAC name is: Hexan-3-one
2. Consider the word root. It is hex. So there are 6 carbon atoms
3. Consider the suffix. It is an. So the ketone is derived from an alkane
• So all the carbon-carbon bonds are single bonds
♦ alkane: ane minus e gives an
♦ alkene: ene minus e gives en
♦ alkyne: yne minus e gives yn
4. So we have 6 carbon atoms with single bonds between them.
• Note that this 'total 6 numbers' is including the C atom in the ㅡCOㅡ group.
5. So we can write:
C ㅡ C ㅡ C ㅡ C ㅡ C ㅡ C
6. The functional group is at the position 3. That is., 'carbon in the functional group' is at the position 3. So we can write:
C ㅡ C ㅡ CO ㅡ C ㅡ C ㅡ C
7. Fill all the valencies of carbon atoms.
• We use hydrogen to fill up the valencies.
• The result is: CH3ㅡCH2ㅡCOㅡCH2ㅡCH2ㅡCH3
■ In the first section in this chapter, we saw how the 'alkyl radical' gets itself attached to an open chain. See details here.
■ In another previous section we saw how a 'hydroxyl group' that is., 'ㅡOH', gets itself attached to an open chain. See details here.
■ In the just previous section we saw how an 'aldehyde group' that is., 'ㅡCHO', gets itself attached to an open chain. See details here.
■ Now we will see the same for the keto group:
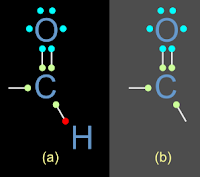
• We have seen the structure of ㅡCHO group in the previous section. It is shown again in fig.14.70 (a) below:
 |
| Fig.14.70 |
♦ This need is indicated by the absence of a dot at the end of the bond line attached to the C atom.
• Now consider fig.14.70(b). It shows the bonding details in the ㅡCOㅡ group
♦ All the valency needs of oxygen atoms is satisfied (6 cyan dots plus two green dots, giving a total of 8 dots.
♦ Those of carbon is not fully satisfied. (4 green plus 2 cyan dots give a total of only 6)
• The need for two electrons is indicated by the absence of a dot at the end of each of the two bond lines attached to the C atom.
♦ Thus the group as a whole, is in need for two electrons.
• This need will be satisfied when the group gets itself attached to a hydrocarbon chain as shown in fig.14.71 below:
 |
| Fig.14.71 |
• In fig.14.71, the group ㅡCOㅡ is flanked on the left by ethyl group and on the right by methyl group. So we can write: CH3ㅡCH2ㅡCOㅡCH3.
• It is Butan-2-one
In the next section, we will see the Carboxylic acid group.
No comments:
Post a Comment