In the previous section, we completed the discussion on the Periodic table. In this section we will discuss about Non-metals. We will
discuss about non-metals like oxygen, hydrogen, nitrogen, chlorine
etc., For each of these non-metals, we will see:
In the next section, we will discuss the reaction of oxygen with metals.
• Uses • Importance • Occurrence in nature • Preparation in the lab.
First we will see oxygen:
Oxygen is a gas. It is present
in the atmosphere. But in the atmosphere, there are lots of other
gases also. If we take a sample of air from the atmosphere,
• 78.08% of that sample will be
nitrogen • 20.95% will be oxygen • 0.9% will be argon • 0.038% will be carbon dioxide
• The remaining portion, that is
[100 -(78.08 +20.95 +0.9 +0.038)] 0.032% will consist of other gases.
So we see that oxygen has a
significant presence. It is the breath of life. It is most essential
for the existence of life. We know that oxygen used up by living
beings is replenished by plants.
Oxygen was discovered by a British scientist Joseph Priestly. It was identified as an element by a French scientist Antoine Lavoisier. The word 'oxygen' comes from a Greek word that means 'acid former'.
Preparation of oxygen in the
lab:
• In the lab, oxygen is prepared
by heating crystals of potassium permanganate in a dry ‘boiling tube’.
• Note that the ‘boiling tube’ must be dry. That is.,
there must not be any presence of water or other substances.
The diagram of the process is shown in fig.5.1 below. [It may be noted that, all experiments should be carried out under the supervision of teachers. All safety precautions should be taken]
 |
| Fig.5.1 |
■ So what happens when the
crystals are heated?
• The crystals break down into
three new substances:
(i) Potassium manganate: K2MnO4
(ii) Manganese dioxide MnO2 and
(iii) Oxygen: O2
• So we see that oxygen is one
of the ‘three new substances’. It is in the gaseous form, and so
will rise towards the top of the tube.
• If we introduce a glowing splinter into the tube, it will burst into flame. This will tell us
about the presence of oxygen. A video can be seen here.
■ So we tested and confirmed the
presence of oxygen using the glowing splinter. How do we know the
presence of the other two: K2MnO4 and MnO2
?
• There are various tests and
procedures to determine the contents in the tube after the reaction.
Such tests and procedures are used both in labs and in industries. We
will learn about them in higher classes. For the present discussion,
it is sufficient to know which are the 'three new substances'.
So the crystals of potassium
permanganate changed into three different substances. It is a
decomposition reaction. As heat is required for the decomposition to
take place, it is called a thermal decomposition reaction.
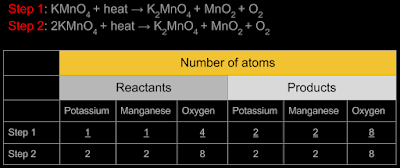
1. Let us write the equation
for the reaction:
KMnO4
+ heat → K2MnO4
+ MnO2
+ O2
2. Let us analyse the above
equation. When we take some crystals of potassium permanganate in the
tube, we are taking some molecules of it.
3.Any one molecule of
potassium permanganate is KMnO4.
So this molecule is placed on the left side of the equation in (1).
4. When heat is applied, we
get three ‘new substances’.
• One molecule of a new
substance is K2MnO4.
So it is placed on the right side of the equation in (1)
• One molecule of another
new substance is MnO2.
So it is also placed on the right side of the equation in (1)
• One molecule of the third new substance is O2.
So it is also placed on the right side of the equation in (1)
5. The equation seems to be
complete. But it is not. Writing such an equation will not give us
the ‘number of molecules’ of each substance involved in the
reaction. [Equation in (1) is called the 'skeletal equation']. So we must write a balanced
equation. Details here.
7. So the balanced equation is: 2KMnO4
+ heat → K2MnO4
+ MnO2
+ O2
From the balanced equation, we can see that, 2 molecules of potassium permanganate will be required to produce one molecule of oxygen.
8. Also, from balanced equations, we can calculate accurate quantities of substances taking part in a reaction. We will learn more about calculation of quantities in higher classes.
8. Also, from balanced equations, we can calculate accurate quantities of substances taking part in a reaction. We will learn more about calculation of quantities in higher classes.
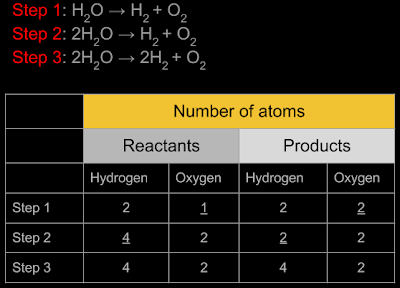
Another method for the
preparation of oxygen:
1. During electrolysis of water,
we get hydrogen and oxygen. So it can also be used for the
preparation of oxygen. Let us analyse the equation:
2. The original substance that we
take is water. So we will write one molecule of it on the left side.
3. The new substances that we get
are hydrogen and oxygen. So we will write one molecule of each on the
right side. So the skeletal equation is:
H2O
→ H2 +
O2
5. So we see that oxygen can be
prepared by the electrolysis of water also.
Chemical reactions in which oxygen is one of the reactants
Reaction of oxygen with other non-metals:
Take a small quantity of sulphur in a spatula and burn it. We will get the smell of sulphur dioxide.
Take a small quantity of sulphur in a spatula and burn it. We will get the smell of sulphur dioxide.
Sulphur dioxide is a gas. It
is formed when sulphur is burnt. During the burning, a chemical
reaction takes place. The reaction is between sulphur and oxygen.
■ A process of burning a substance in oxygen is called combustion.
■ A process of burning a substance in oxygen is called combustion.
So we see that sulphur and
oxygen reacts together to form sulphur dioxide. Let us write the
equation:
• One molecule of sulphur is S. One molecule of oxygen is O2. We will write them on the left side.
• One molecule of sulphur dioxide is SO2. We will write it on the right side.
• So the skeletal equation is: S + O2 → SO2 . This equation is already balanced.
• One molecule of sulphur is S. One molecule of oxygen is O2. We will write them on the left side.
• One molecule of sulphur dioxide is SO2. We will write it on the right side.
• So the skeletal equation is: S + O2 → SO2 . This equation is already balanced.
■ Sulphur is a non-metal. So
this is an example of the reaction of oxygen with a non-metal. There
are more examples in which oxygen reacts with other non-metals:
■ Reaction with carbon: Carbon burns in oxygen to form carbon dioxide. When the burning takes place, the carbon is reacting with oxygen.
• We will write one molecule each of carbon and oxygen on the left side
• We will write one molecule of the product, which is carbon dioxide, on the right side
• So the skeletal equation is: C + O2 → CO2 . This skeletal equation is already balanced.
■ Reaction with hydrogen: Hydrogen and oxygen reacts together to form water. But the reaction can be carried out only in special labs with special equipments. It is an explosive reaction, and so must be carried out only under authorised technical supervision.
• We will write one molecule each of hydrogen and oxygen on the left side
• We will write one molecule of the product, which is water, on the right side
• So the skeletal equation is: H2 + O2 → H2O.
• This is not a balanced equation. The steps for writing the balanced equation is shown below:
• So the balanced equation is: 2H2 + O2 → 2H2O.
• From the balanced equation, we can see that 2 molecules of hydrogen will be required to react with one molecule of oxygen to produce water. Also note that, the reaction will give two molecules of water as the product.
■ Reaction with carbon: Carbon burns in oxygen to form carbon dioxide. When the burning takes place, the carbon is reacting with oxygen.
• We will write one molecule each of carbon and oxygen on the left side
• We will write one molecule of the product, which is carbon dioxide, on the right side
• So the skeletal equation is: C + O2 → CO2 . This skeletal equation is already balanced.
■ Reaction with hydrogen: Hydrogen and oxygen reacts together to form water. But the reaction can be carried out only in special labs with special equipments. It is an explosive reaction, and so must be carried out only under authorised technical supervision.
• We will write one molecule each of hydrogen and oxygen on the left side
• We will write one molecule of the product, which is water, on the right side
• So the skeletal equation is: H2 + O2 → H2O.
• This is not a balanced equation. The steps for writing the balanced equation is shown below:
• So the balanced equation is: 2H2 + O2 → 2H2O.
• From the balanced equation, we can see that 2 molecules of hydrogen will be required to react with one molecule of oxygen to produce water. Also note that, the reaction will give two molecules of water as the product.
In the next section, we will discuss the reaction of oxygen with metals.

No comments:
Post a Comment