In the previous section, we saw how to determine the chemical formula of any compound from the valency of it's component elements. In this section, we will see Oxidation and Reduction.
• After the reaction, Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+.
♦ The process of losing the two electrons can be written as: Mg → Mg2+ + 2e-
• Each Cl has become a negative ion. This is due to the gain of electron. One electrons is gained by each Cl. So the charge is 1- for each.
♦ The process of gaining electrons can be written as: 2Cl + 2e- → 2Cl-
• In the reaction, Mg donates electrons, and, Cl accepts electrons.
■ Oxidation is the process of donation of electrons
■ Reduction is the process of accepting electrons
Is the word 'oxidation' related to 'oxygen' in any way?
To find the answer, we must look into some history.
• The term 'oxidation' was first used by Lavoisier to mean 'reaction of a substance with oxygen'.
• Much later, it was realized that, when any element 'X' reacts with oxygen (that is., when 'X' is oxidised), electrons are donated by 'X'
• So later, the term 'oxidation' began to be applied to all elements which donates electrons in a reaction. Regardless of whether it's reaction is with oxygen, or not.
And the opposite is applicable to oxidation:
The element which donates electrons can be considered to be 'oxidised'
So when we see Na+1Cl-1 , we can write the following details:
• Na is in an oxidation state of +1. That is., it has lost one electron
• Cl is in an oxidation state of -1. That is., it has gained one electron
Let us consider some more examples:
Consider MgO. We have seen the details here. The diagram is shown again below:
We can write the oxidation state as: Mg+2O-2
• Mg is in an oxidation state of +2. That is., it has lost two electrons
• O is in an oxidation state of -2. That is., it has gained two electrons
Consider Aluminium chloride (AlCl3)
• Al, with atomic number 13, has the electronic configuration 2,8,3 (See here)
• Cl, with atomic number 17, has the electronic configuration 2,8,7
• So Al needs to lose 3 electrons and Cl needs to gain one electron
• Thus 3 chlorine atoms combine with one aluminium atom. Each chlorine atom accept one of the three electrons donated by the aluminium atom
• One aluminium atom loses 3 electrons. So it's oxidation state is +3
• Each chlorine atom gains one electron so oxidation state of each is -1
• So we can write: Al+3Cl3-1
When we see Al+3Cl3-1, we can write the following:
• Aluminium is in an oxidation state of +3. That is., it has lost 3 electrons
• Chlorine is in an oxidation state of -1. That is., each chlorine atom has gained one electron
For covalent compounds, there is no transfer of electrons. Only sharing of electrons takes place. That is., there is no donating or accepting of electrons. In such a case, how do we write the oxidation number?
Consider HCl. We have seen the details here. The diagram is shown again below:
In such cases, it is assumed that, the shared pairs of electrons are completely displaced towards the more electronegative atom. Let us see the application of this assumption in our present case.
• Electronegativity of H = 2.20 (see the chart here)
• Electronegativity of Cl = 3.16
• Chlorine is more electronegative. So the shared pair of electrons move towards the chlorine atom.
• That is., two electrons move towards the chlorine atom
• Out of those two, one was already possessed by Cl. So the number of extra electrons is only 1
• Thus the chlorine is given an oxidation number of -1
• What about hydrogen?
• The hydrogen is now left with zero electrons. Because the pair as a whole, has moved away from the hydrogen atom
• Out of the two electrons that has moved away, one belonged to the hydrogen atom. Now it is gone.
• So in effect, hydrogen has lost one electron, and so it is given an oxidation number of +1
• We can write the equation as: H20 + Cl20 → 2H+1Cl-1
Let us see another example. Consider carbon dioxide CO2
Two oxygen atoms forms a covalent bond with carbon. We saw the details here. It is shown again below:
• Electronegativity of C = 2.55
• Electronegativity of O = 3.44
• Oxygen is more electronegative. So the shared pair of electrons move towards the oxygen atom.
• That is., in each bond, four electrons move towards the oxygen atom
• Out of those four, two were already possessed by O. So the number of extra electrons is only 2
• Thus each oxygen is given an oxidation number of -2
• What about carbon?
• The carbon is now left with zero electrons. Because both the pairs have moved away from the carbon atom
• Out of the eight electrons that has moved away, four belonged to the carbon atom. Now they are gone.
• So in effect, carbon has lost four electrons, and so, it is given an oxidation number of +4
• We can write the equation as: C0 + 2O0 → C+4O2-2
So we see that for both ionic and covalent compounds, the component elements will have oxidation numbers. But it is very important to remember an exception to this rule:
Consider the formation of HCl that we saw above. If we examine the equation H20 + Cl20 → 2H+1Cl-1 , we will see that:
• The oxidation number of H has increased from 0 to +1
• The oxidation number of Cl has decreased from 0 to -1
■ The element, whose oxidation number increases, is oxidised. In other words, the element, whose oxidation number increases, has undergone oxidation.
♦ In our example, hydrogen is oxidised. In other words, hydrogen has undergone oxidation
■ The element, whose oxidation number decreases, is reduced. In other words, the element, whose oxidation number decreases, has undergone reduction.
♦ In our example, chlorine is reduced. In other words, chlorine has undergone reduction.
Another example: Consider the formation of carbon dioxide. If we examine the equation C0 + 2O0 → C+4O2-2 , we will see that:
• The oxidation number of C has increased from 0 to +4. So C has undergone oxidation
• The oxidation number of O has decreased from 0 to -2. So O has undergone reduction
We see that, in a reaction, one element is oxidised and the other element is reduced. That is., oxidation and reduction takes place simultaneously. So the overall process is known as a redox process.
We see that, in a reaction, the element which increases in the oxidation number is the one which gets oxidised.
• That element gets oxidised by whom?
• It is oxidised by the other element. We know that, this 'other element' which causes the oxidation is called the 'oxidising agent'.
• Now, what is the state of the 'oxidation number' of this 'other element', which is the 'oxidising agent'?
• The 'oxidation number' of this 'other element' decreases.
■ So we can write: The element whose oxidation number decreases, is the oxidising agent.
■ Similarly, The element whose oxidation number increases is the reducing agent
This can be explained using a general example. Consider the reaction between two elements 'P' and 'Q' (symbols are not real):
• Px1 + Qy1 → Px2Qy2 [(x2 > x1) and (y2 <y1)]
• We see that, after the reaction, P has an increased oxidation number.
• So P has undergone oxidation
• Obviuosly, this oxidation was caused by Q, and so, we call Q, the oxidising agent
• Oxidation number of Q has decreased
■ So we can write: The element whose oxidation number decreases, is the oxidising agent.
And the reverse can also be worked out:
• We see that, after the reaction, Q has a decreased oxidation number.
• So Q has undergone reduction
• Obviuosly, this reduction was caused by P, and so, we call P, the reducing agent
• Oxidation number of P has increased
• So we can write: The element whose oxidation number increases, is the reducing agent.
We will now see an example:
• We are able to find the above because, the 'oxidation number' of one element increases, and that of the other element decreases.
• But there is some thing more than just 'increase and decrease':
■ The sum of oxidation numbers in a compound will be always zero.
Let us see an example. Consider Zn+2Cl2-1:
• Total oxidation number of Zn:
♦ There is only one Zn atom, and it has an oxidation number of +2. Thus:
♦ Total oxidation number of Zn = number of Zn atoms × oxidation number of each = 1 × +2 = +2
• Total oxidation number of Cl:
♦ There are two Cl atoms, each with an oxidation number -1. Thus:
♦ Total oxidation number of Cl = number of Cl atoms × oxidation number of each = 2 × -1 = -2
■ Total sum of the oxidation numbers of all the atoms in the molecule = 2 + -2 = 0
Another example: Consider Al+3Cl3-1:
• Total oxidation number of Al:
♦ There is only one Al atom, and it has an oxidation number of +3. Thus:
♦ Total oxidation number of Al = number of Al atoms × oxidation number of each = 1 × +3 = +3
• Total oxidation number of Cl:
♦ There are three Cl atoms, each with an oxidation number -1. Thus:
♦ Total oxidation number of Cl = number of Cl atoms × oxidation number of each = 3 × -1 = -3
■ Total sum of the oxidation numbers of all the atoms in the molecule = 3 + -3 = 0
Another example: Consider Mg+2O-2:
• Total oxidation number of Mg:
♦ There is only one Mg atom, and it has an oxidation number of +2. Thus:
♦ Total oxidation number of Mg = number of Mg atoms × oxidation number of each = 1 × +2 = +2
• Total oxidation number of O:
♦ There is only one O atom, and it has an oxidation number of -2. Thus:
♦ Total oxidation number of O = number of O atoms × oxidation number of each = 1 × -2 = -2
■ Total sum of the oxidation numbers of all the atoms in the molecule = 2 + -2 = 0
Thus we find that the sum of oxidation numbers in any molecule is equal to zero. In the next section, we will see a practical application of this property
Oxidation and Reduction
We have seen the reaction between magnesium and chlorine. (Details here). The electron dot diagram is shown again below:• After the reaction, Mg has become a positive ion. This is due to the loss of electrons. Two electrons are lost. So the charge is 2+.
♦ The process of losing the two electrons can be written as: Mg → Mg2+ + 2e-
• Each Cl has become a negative ion. This is due to the gain of electron. One electrons is gained by each Cl. So the charge is 1- for each.
♦ The process of gaining electrons can be written as: 2Cl + 2e- → 2Cl-
• In the reaction, Mg donates electrons, and, Cl accepts electrons.
■ Oxidation is the process of donation of electrons
■ Reduction is the process of accepting electrons
To find the answer, we must look into some history.
• The term 'oxidation' was first used by Lavoisier to mean 'reaction of a substance with oxygen'.
• Much later, it was realized that, when any element 'X' reacts with oxygen (that is., when 'X' is oxidised), electrons are donated by 'X'
• So later, the term 'oxidation' began to be applied to all elements which donates electrons in a reaction. Regardless of whether it's reaction is with oxygen, or not.
An analogy to easily remember the 'difference between oxidation and reduction' is as follows:
When electrons are accepted, negative charge increases. 'Increasing negative' can be considered as 'decreasing (reducing) value'. So the element which accepts electrons can be considered to be 'reduced'.And the opposite is applicable to oxidation:
The element which donates electrons can be considered to be 'oxidised'
• So in the above reaction, chlorine is reduced. Reduced by whom?
♦ By magnesium. Magnesium reduces chlorine by 'forcing chlorine to accept electrons'. So magnesium is the reducing agent
The reverse can also be written:
• Magnesium is oxidised. Oxidised by whom?
♦ By chlorine. Chlorine oxidises magnesium by 'forcing magnesium to donate electrons'. So chlorine is the oxidising agent.
Now let us see one more example. That of Sodium fluoride (NaF):
• NaF is produced by the reaction between sodium (Na) and fluorine (F)
• Na, with atomic number 11, has the electronic configuration 2,8,1 (See here)
• F, with atomic number 9, has the electronic configuration 2,7
• So during the reaction, Na donates one electron, and, F accepts one electron
• Thus we say: Na is oxidised, and, F is reduced
• Na is oxidised by F. Because, F forces Na, to donate one electron. So F is oxidising agent
• F is reduced by Na. Because, Na forces F, to accept one electron. So Na is the reducing agent
• NaF is produced by the reaction between sodium (Na) and fluorine (F)
• Na, with atomic number 11, has the electronic configuration 2,8,1 (See here)
• F, with atomic number 9, has the electronic configuration 2,7
• So during the reaction, Na donates one electron, and, F accepts one electron
• Thus we say: Na is oxidised, and, F is reduced
• Na is oxidised by F. Because, F forces Na, to donate one electron. So F is oxidising agent
• F is reduced by Na. Because, Na forces F, to accept one electron. So Na is the reducing agent
Oxidation number
Every element in a compound will get a particular number. What is this number? How is it determined? What is it's significance? Does this number help us in any way? Let us see the answers:
• This number is called the oxidation number
• Upon seeing the oxidation number of an element in a compound, we will immediately be able to say how many electrons that element has gained (or lost)
• For example, in NaCl, the Na atom has lost one electron. So it is given an oxidation number: +1
(Why 'positive' one? The answer is simple: losing electrons means gaining positive charge)
• Similarly, the Cl atom has gained one electron. So it is given an oxidation number: -1
• Together, they are represented as: Na+1Cl-1
• Together, they are represented as: Na+1Cl-1
So when we see Na+1Cl-1 , we can write the following details:
• Na is in an oxidation state of +1. That is., it has lost one electron
• Cl is in an oxidation state of -1. That is., it has gained one electron
Let us consider some more examples:
Consider MgO. We have seen the details here. The diagram is shown again below:
We can write the oxidation state as: Mg+2O-2
• Mg is in an oxidation state of +2. That is., it has lost two electrons
• O is in an oxidation state of -2. That is., it has gained two electrons
Consider Aluminium chloride (AlCl3)
• Al, with atomic number 13, has the electronic configuration 2,8,3 (See here)
• Cl, with atomic number 17, has the electronic configuration 2,8,7
• So Al needs to lose 3 electrons and Cl needs to gain one electron
• Thus 3 chlorine atoms combine with one aluminium atom. Each chlorine atom accept one of the three electrons donated by the aluminium atom
• One aluminium atom loses 3 electrons. So it's oxidation state is +3
• Each chlorine atom gains one electron so oxidation state of each is -1
• So we can write: Al+3Cl3-1
When we see Al+3Cl3-1, we can write the following:
• Aluminium is in an oxidation state of +3. That is., it has lost 3 electrons
• Chlorine is in an oxidation state of -1. That is., each chlorine atom has gained one electron
For covalent compounds, there is no transfer of electrons. Only sharing of electrons takes place. That is., there is no donating or accepting of electrons. In such a case, how do we write the oxidation number?
Consider HCl. We have seen the details here. The diagram is shown again below:
In such cases, it is assumed that, the shared pairs of electrons are completely displaced towards the more electronegative atom. Let us see the application of this assumption in our present case.
• Electronegativity of H = 2.20 (see the chart here)
• Electronegativity of Cl = 3.16
• Chlorine is more electronegative. So the shared pair of electrons move towards the chlorine atom.
• That is., two electrons move towards the chlorine atom
• Out of those two, one was already possessed by Cl. So the number of extra electrons is only 1
• Thus the chlorine is given an oxidation number of -1
• What about hydrogen?
• The hydrogen is now left with zero electrons. Because the pair as a whole, has moved away from the hydrogen atom
• Out of the two electrons that has moved away, one belonged to the hydrogen atom. Now it is gone.
• So in effect, hydrogen has lost one electron, and so it is given an oxidation number of +1
• We can write the equation as: H20 + Cl20 → 2H+1Cl-1
Let us see another example. Consider carbon dioxide CO2
Two oxygen atoms forms a covalent bond with carbon. We saw the details here. It is shown again below:
• Electronegativity of C = 2.55
• Electronegativity of O = 3.44
• Oxygen is more electronegative. So the shared pair of electrons move towards the oxygen atom.
• That is., in each bond, four electrons move towards the oxygen atom
• Out of those four, two were already possessed by O. So the number of extra electrons is only 2
• Thus each oxygen is given an oxidation number of -2
• What about carbon?
• The carbon is now left with zero electrons. Because both the pairs have moved away from the carbon atom
• Out of the eight electrons that has moved away, four belonged to the carbon atom. Now they are gone.
• So in effect, carbon has lost four electrons, and so, it is given an oxidation number of +4
• We can write the equation as: C0 + 2O0 → C+4O2-2
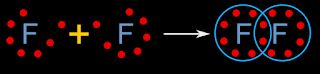
• Consider the molecules of elements. In such molecules, the component atoms are all of the same element.
• All the component atoms will be having the same power to attract the shared electrons. We have seen examples of such bonding here. Some are shown again below:
• So we see that, when the component atoms are the same, the shared electron pairs will be attracted equally, and so they will remain exactly midway between the atoms
• No atom will get any extra electrons, and so the oxidation numbers of all component atoms will be zero
• We write them as: F20, Cl20 etc.,
Telling 'whether it is an oxidation or a reduction' by using oxidation number
So we have seen the oxidation number of elements in both ionic and covalent compounds. We will now see a practical application of this number:Consider the formation of HCl that we saw above. If we examine the equation H20 + Cl20 → 2H+1Cl-1 , we will see that:
• The oxidation number of H has increased from 0 to +1
• The oxidation number of Cl has decreased from 0 to -1
■ The element, whose oxidation number increases, is oxidised. In other words, the element, whose oxidation number increases, has undergone oxidation.
♦ In our example, hydrogen is oxidised. In other words, hydrogen has undergone oxidation
■ The element, whose oxidation number decreases, is reduced. In other words, the element, whose oxidation number decreases, has undergone reduction.
♦ In our example, chlorine is reduced. In other words, chlorine has undergone reduction.
Another example: Consider the formation of carbon dioxide. If we examine the equation C0 + 2O0 → C+4O2-2 , we will see that:
• The oxidation number of C has increased from 0 to +4. So C has undergone oxidation
• The oxidation number of O has decreased from 0 to -2. So O has undergone reduction
We see that, in a reaction, one element is oxidised and the other element is reduced. That is., oxidation and reduction takes place simultaneously. So the overall process is known as a redox process.
We see that, in a reaction, the element which increases in the oxidation number is the one which gets oxidised.
• That element gets oxidised by whom?
• It is oxidised by the other element. We know that, this 'other element' which causes the oxidation is called the 'oxidising agent'.
• Now, what is the state of the 'oxidation number' of this 'other element', which is the 'oxidising agent'?
• The 'oxidation number' of this 'other element' decreases.
■ So we can write: The element whose oxidation number decreases, is the oxidising agent.
■ Similarly, The element whose oxidation number increases is the reducing agent
This can be explained using a general example. Consider the reaction between two elements 'P' and 'Q' (symbols are not real):
• Px1 + Qy1 → Px2Qy2 [(x2 > x1) and (y2 <y1)]
• We see that, after the reaction, P has an increased oxidation number.
• So P has undergone oxidation
• Obviuosly, this oxidation was caused by Q, and so, we call Q, the oxidising agent
• Oxidation number of Q has decreased
■ So we can write: The element whose oxidation number decreases, is the oxidising agent.
And the reverse can also be worked out:
• We see that, after the reaction, Q has a decreased oxidation number.
• So Q has undergone reduction
• Obviuosly, this reduction was caused by P, and so, we call P, the reducing agent
• Oxidation number of P has increased
• So we can write: The element whose oxidation number increases, is the reducing agent.
• The equation for the reaction between hydrochloric acid (HCl) and zinc (Zn), to form zinc chloride and hydrogen is given below:
• Zn + 2HCl → ZnCl2 + H2
• Note that, it is a balanced equation. The modified form of this equation, which shows the oxidation number of each element is given below:
• Zn0 + 2H+1Cl-1→ Zn+2Cl2-1 + H20
• From this modified equation, we can write the following details:
■ Oxidation number of Zn increases from 0 to +2. So, Zn undergoes oxidation. And also, it is the reducing agent
■ Oxidation number of H decreases from +1 to 0. So H undergoes reduction. And also, it is the oxidising agent
• So now we are able to find which element gets oxidised, and which element gets reduced
• Also, we can find which is the oxidising agent, and which is the reducing agent• We are able to find the above because, the 'oxidation number' of one element increases, and that of the other element decreases.
• But there is some thing more than just 'increase and decrease':
■ The sum of oxidation numbers in a compound will be always zero.
Let us see an example. Consider Zn+2Cl2-1:
• Total oxidation number of Zn:
♦ There is only one Zn atom, and it has an oxidation number of +2. Thus:
♦ Total oxidation number of Zn = number of Zn atoms × oxidation number of each = 1 × +2 = +2
• Total oxidation number of Cl:
♦ There are two Cl atoms, each with an oxidation number -1. Thus:
♦ Total oxidation number of Cl = number of Cl atoms × oxidation number of each = 2 × -1 = -2
■ Total sum of the oxidation numbers of all the atoms in the molecule = 2 + -2 = 0
Another example: Consider Al+3Cl3-1:
• Total oxidation number of Al:
♦ There is only one Al atom, and it has an oxidation number of +3. Thus:
♦ Total oxidation number of Al = number of Al atoms × oxidation number of each = 1 × +3 = +3
• Total oxidation number of Cl:
♦ There are three Cl atoms, each with an oxidation number -1. Thus:
♦ Total oxidation number of Cl = number of Cl atoms × oxidation number of each = 3 × -1 = -3
■ Total sum of the oxidation numbers of all the atoms in the molecule = 3 + -3 = 0
Another example: Consider Mg+2O-2:
• Total oxidation number of Mg:
♦ There is only one Mg atom, and it has an oxidation number of +2. Thus:
♦ Total oxidation number of Mg = number of Mg atoms × oxidation number of each = 1 × +2 = +2
• Total oxidation number of O:
♦ There is only one O atom, and it has an oxidation number of -2. Thus:
♦ Total oxidation number of O = number of O atoms × oxidation number of each = 1 × -2 = -2
■ Total sum of the oxidation numbers of all the atoms in the molecule = 2 + -2 = 0
Thus we find that the sum of oxidation numbers in any molecule is equal to zero. In the next section, we will see a practical application of this property





No comments:
Post a Comment